Abstract
Background: The FMS-like tyrosine kinase 3 (FLT3) mutations occur in approximately 30% of acute myeloid leukemia (AML) patients, which are associated with worse survival and higher relapse risk. XY0206 is a novel oral FLT3 inhibitor with preclinical activity against FLT3 internal tandem duplication mutations (FLT3-ITD). The Phase Ⅰ/Ⅱ clinical trail with XY0206 (NCT04471064) is currently underway to explore the initial efficacy, safety and pharmacokinetic (PK) of XY0206 in patients with relapsed/refractory AML (R/R AML).
Methods: This open-label, multicenter, dose-escalation and dose-expansion Phase Ⅰ/Ⅱ trail enrolled subjects from August 2020 to September 2022 who were aged ≥18 years with R/R AML. Six dose-escalation or dose-expansion cohorts (12.5, 25, 37.5, 50, 62.5 mg per day, or 25 mg twice a day) were set up to explore the safety/tolerability and antileukemic activity of XY0206, and to determine the maximal tolerated dose (MTD) of XY0206. Safety and tolerability were assessed by monitoring dose-limiting toxicities (DLT), treament-emergent adverse events (TEAE) and adverse drug reaction (ADR). Cohort expansion was based on safety and antileukemic activity.
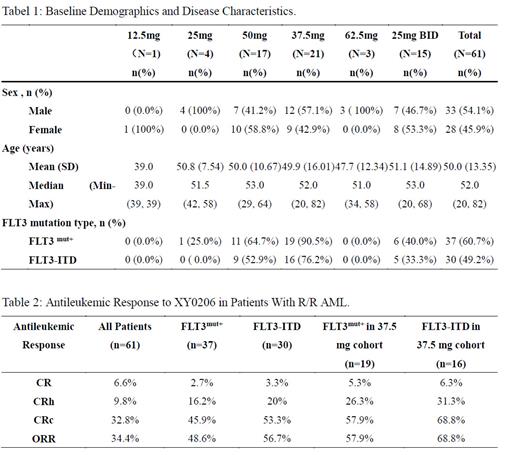
Results: As of December 14, 2022, a total of 61 subjects were enrolled (37 with FLT3 mutation-positive). Demographics and baseline characteristics of patients are presented in Table 1. MTD of XY0206 was established as 50 mg when two of the three patients enrolled in the 62.5 mg dose-escalation cohort could not tolerate continuous medication. The 37.5 mg, 50 mg and 25 mg BID dose cohorts were further expanded. Antileukemic activity was assessed in the Full Analysis Set (FAS, n=61), with overall response rate (ORR) was 34.4% (n=21/61), composite complete remission (CRc) was 32.8% (n=20/61), complete remission (CR) was 6.6% (n=4/61) and CR with partial hematologic recovery (CRh) was 9.8% ( n=6/61 ). Among the patients with FLT3 mutation-positive (FLT3 mut+, n= 37), ORR was 48.6%(n=18/37), CRc was 45.9% ( n=17/37), CR was 2.7% (n=1/37) and CRh was 16.2% (n=6/37). Among the patients with FLT3-ITD mutation (n=30), ORR was 56.7% (n=17/30), CRc was 53.3% (n=16/30), CR was 3.3% (n=1/30) and CRh was 20%( n=6/30). The 37.5 mg dose cohort as the target dose was further expanded to include FLT3 mut+ patients only. CRc in FLT3 mut+ patients ( n= 19 ) and FLT3-ITD mutation patients (n=16) was 57.9% (n=11/19) and 68.8% (n=11/16 ), CR was 5.3% (n=1/19) and 6.3% (n=1/16), CRh was 26.3% (n=5/19 ) and 31.3% (n=5/16), respectively. In terms of safety, the overall incidence and severity of TEAE/ADR in 61 subjects did not correlate with dose significantly. The most common grade 3 and above adverse events (≥ 50% ) were leukopenia (65.6% ), thromobocytopenia (63.9%), neutropenia (62.3%), animia (52.5%) and hypokalemia (50.8%). The fatal adverse event was disease progression, which was mostly related to AML.
Conclusion: XY0206 is a novel FLT3 inhibitor that showed a good safety profile, a potent anti-leukemic activity and improved efficacy in patients with FLT3 mut+ R/R AML.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal